Answer:
The theoretical yield of lithium nitride is 7.52 g.
Step-by-step explanation:
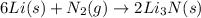
Moles of lithium =
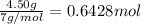
Moles of nitrogen gas =
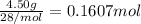
According to reaction, 6 moles of lithium reacts with 1 mole of nitrogen gas.
Then 0.6428 moles of lithium will react with:
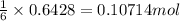 of nitrogen gas
of nitrogen gas
As we can see that moles of lithium are in limiting amount. So, the moles of lithium nitride will be depend upon the moles of lithium. Hence, lithium is a limiting reagent.
According to reaction,6 moles of lithium gives 2 moles of lithium nitride.
Then 0.6428 moles of lithium will give:
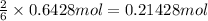 of lithium nitride
of lithium nitride
Mass of 0.21428 moles of lithium nitride:
0.2143 mol × 35 g/mol= 7.52 g