Answer : The hydronium ion concentration and the pH of the solution is,
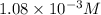 and 2.97 respectively.
and 2.97 respectively.
Solution : Given,
Concentration (c) = 0.0020 M
Acid dissociation constant =
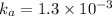
The equilibrium reaction for dissociation of
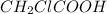 (weak acid) is,
(weak acid) is,
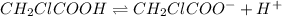
initially conc. c 0 0
At eqm.
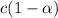
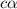
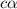
First we have to calculate the concentration of value of dissociation constant
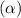 .
.
Formula used :
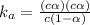
Now put all the given values in this formula ,we get the value of dissociation constant
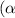 .
.
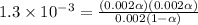
By solving the terms, we get
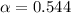
Now we have to calculate the concentration of hydronium ion or hydrogen ion.
![[H^+]=c\alpha=0.002* 0.544=1.08* 10^(-3)M](https://img.qammunity.org/2020/formulas/chemistry/college/lukfm65ie4ombf2kv2hhoqmv5mklgtqj3h.png)
Now we have to calculate the pH.
![pH=-\log [H^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ipfjz05f4cfbguiwup37xvxa7furlbuapf.png)
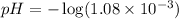
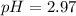
Therefore, the pH of the solution is, 2.97