Answer:
(A) -117.48°C (B) 80.5368°C
Step-by-step explanation:
It is given that density of ethanol is 0.789
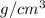
volume of ethanol=89.8 mL
mass of ethanol = density of ethanol×volume = 0.789×89.8 = 70.8522 g
molar mass of ethylene glycol = 62 g/mole
therefore moles of ethylene glycol in 7.6 g of it = 7.7/62 = 0.1241 mole
molality = moles of ethylene glycol/mass of ethanol in kg
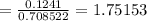
also Kf for ethanol = 1.99 C/m
And Kb for ethanol = 1.22 C/m
boiling point of ethanol = 78.4 °C
freezing point of ethanol = -114° C
(A) depression in freezing point = molality×Kf = 1.75153×1.99 = 3.4855
thus freezing point of solution = -114-3.4855= -117.48°C
(B) elevation in boiling point = molality×Kb = 1.75153×1.22 = 2.13686
thus boiling point of solution = 78.4+2.13686=80.5368°C