Answer:
(a) 22.3 torr; 5.6 torr; (b) 27.9 torr; (c) 77.7 % heptane; 23.3 % octane
(d) Heptane is more volatile than octane
Step-by-step explanation:
We can use Raoult's Law to solve this problem.
It states that the partial pressure of each component of an ideal mixture of liquids is equal to the vapour pressure of the pure component multiplied by its mole fraction. In symbols,
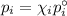
(a) Vapour pressure of each component
Let heptane be Component 1 and octane be Component 2.
(i) Moles of each component
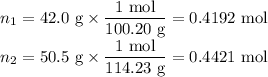
(ii) Total moles
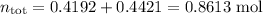
(iiii) Mole fractions of each component
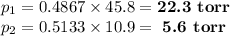
(iv) Partial vapour pressures of each component
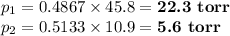
(b) Total pressure
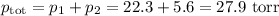
(c) Mass percent of each component in vapour
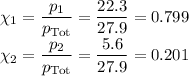
The ratio of the mole fractions is the same as the ratio of the moles.
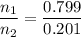
If we have 1 mol of vapour, we have 0.799 mol of heptane and 0.201 mol of octane

(d) Enrichment of vapour
The vapour is enriched in heptane because heptane is more volatile than octane.