Step-by-step explanation:
It is given that,
Initial temperature,
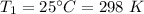
Pressure,
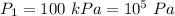
Compression ratio,
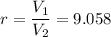
Let T₂ is the final temperature of air. Using the relation for reversible adiabatic process as :
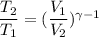 ............(1)
............(1)
Where,
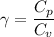
For air,
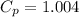 and
and
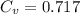
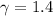
So, equation (1) becomes :
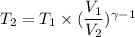
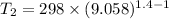
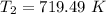
So, the final temperature of air is 719.49 K. Hence, this is the required solution.