Step-by-step explanation:
When emission of industrial gases like sulfur dioxide, carbon dioxide and nitrogen oxide combine with water then the rain that consists of these acids fall into the atmosphere is known as acid rain.
When these gases combine with water then they result in the formation of an acid that is present in an aqueous phase.
So, when sulfur trioxide combines with water then the reaction will be as follows.
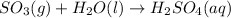
Hence, we can conclude that sulfuric acid is formed when sulfur trioxide gas reacts with water.