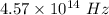Answer:
The frequency of this photon is
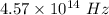
(D) is correct option.
Step-by-step explanation:
Given that,
Excited states,
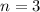
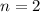
We need to calculate the wavelength
Using formula for energy
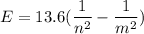
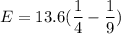
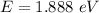
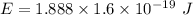
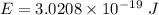
We need to calculate the frequency
Using formula of frequency
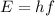
Where, E =energy
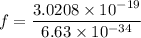

Hence, The frequency of this photon is