Answer:
Vapour pressure of benzene over the solution is 253 torr
Step-by-step explanation:
According to Raoult's law for a mixture of two liquid component A and B-
vapour pressure of a component (A) in solution =

vapour pressure of a component (B) in solution =
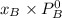
Where
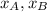 are mole fraction of component A and B in solution respectively
are mole fraction of component A and B in solution respectively
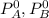 are vapour pressure of pure A and pure B respectively
are vapour pressure of pure A and pure B respectively
Here mole fraction of benzene in solution is 0.340 and vapour pressure of pure benzene is 745 torr
So, vapour pressure of benzene in solution =
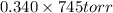
= 253 torr