Answer: c. the molecules with the highest energy evaporate first, lowering the temperature of the sample
Step-by-step explanation:
The process by which liquid starts to change into vapor phase at any temperature is known as evaporation.
During evaporation , the molecules which possess higher energies escape from the upper layer into vapor phase. the molecules which escape draw energy from surroundings and thus decrease the energy of the surroundings and hence lead to decrease in temperature.
As temperature of the system is directly proportional to the energy of the system , thus decrease in energy leads to decrease in temperature.
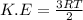
K.E. = Kinetic energy
T = temperature
R= gas constant