Answer:
The kinetic energy of the ejected electrons increases.
Step-by-step explanation:
As we know that electrons are only ejected from a metal surface if the frequency of the incident light increases the work function of the metal. If the frequency of the incident light is less than the work function of the metal no matter how intense the beam the electrons will not be ejected from the surface.
Using conservation of energy principle we have
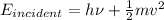
If we increase the intensity of incident light the term on the LHS of the above equation increases this increase appears in the kinetic energy term in RHS of the equation since
 remains constant.
remains constant.