Answer:
444 L
Step-by-step explanation:
According to Archimedes the buoyancy force is equal to the weight of the displaced substance. In this case, the displaced substance is Air. So to calculate the lifting power of Helium, start with the weight of air in volume
Since the Helium itself has weight, we need to use the difference in density between air and helium.
We know that the density of air is 1.2
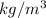
Density of Helium is 0.1787
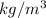
To lift a object of 1 pound which is equal to 0.453 kg we need
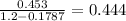

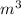 helium There is 1000 liters in a
helium There is 1000 liters in a
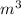
So the required volume of helium= 0.444×1000=444 L