Answer:
Work done,
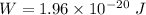
Step-by-step explanation:
It is given that,
Potential difference between the inner and outer surfaces of a cell membrane,
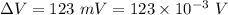
The inner surface is negative relative to the outer surface. Let W is the work required to eject a positive sodium ion (Na+) from the interior of the cell. Work done is given by :
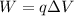
q is the charge on electron
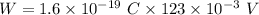
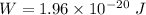
So, the work required to eject a positive sodium ion (Na+) from the interior of the cell is
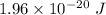 . Hence, this is the required solution.
. Hence, this is the required solution.