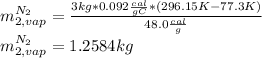Answer:
1.2584kg of nitrogen boils.
Step-by-step explanation:
Consider the energy balance for the overall process. There are not heat or work fluxes to the system, so the total energy keeps the same.
For the explanation, the 1 and 2 subscripts will mean initial and final state, and C and N2 superscripts will mean copper and nitrogen respectively; also, liq and vap will mean liquid and vapor phase respectively.
The overall energy balance for the whole system is:
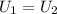
The state 1 is just composed by two phases, the solid copper and the liquid nitrogen, so:
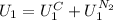
The state 2 is, by the other hand, composed by three phases, solid copper, liquid nitrogen and vapor nitrogen, so:
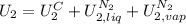
So, the overall energy balance is:
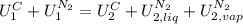
Reorganizing,
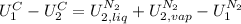
The left part of the equation can be written in terms of the copper Cp because for solids and liquids Cp≅Cv. The right part of the equation is written in terms of masses and specific internal energy:
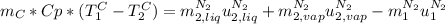
Take in mind that, for the mass balance for nitrogen,
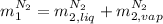 ,
,
So, let's replace
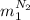 in the energy balance:
in the energy balance:
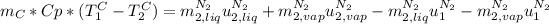
So, as you can see, the term
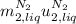 disappear because
disappear because
 (The specific energy in the liquid is the same because the temperature does not change).
(The specific energy in the liquid is the same because the temperature does not change).
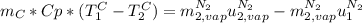

The difference
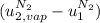 is the latent heat of vaporization because is the specific energy difference between the vapor and the liquid phases, so:
is the latent heat of vaporization because is the specific energy difference between the vapor and the liquid phases, so: