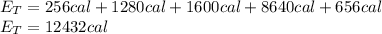Answer:
12432 cal.
Step-by-step explanation:
The process to change ice at -32 ºC to steam at 182 ºC can be divided into 5 steps:
1. Heat the ice to 0 ºC, which is the fusion temperature.
2. Melt the ice (obtaining liquid water), which is a process at constant pressure and temperature, so the liquid obtained is also at 0ºC.
3. Heat the liquid water from 0 ºC to 100 ºC, which is the vaporization normal temperature of the water.
4. Vaporization of all the water; this is also a process that occurs at constant pressure and temperature, so the produced steam will be at 100ºC.
5. Heat the steam from 100 ºC to 182 ºC.
Each process has a required energy, and the sum of the energy required for each and all of the steps is the total amount of energy required for the whole process:
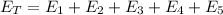
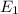 is a heating process for the ice, so we know that the energy required is proportional to the temperature difference through the specific heat:
is a heating process for the ice, so we know that the energy required is proportional to the temperature difference through the specific heat:
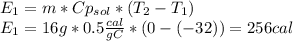
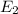 is a phase change process, so we do not use the specific heat (sensible heat), but the fusion heat (latent heat), so:
is a phase change process, so we do not use the specific heat (sensible heat), but the fusion heat (latent heat), so:
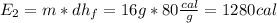
Analogously,
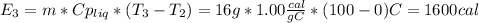
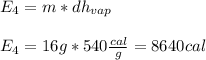
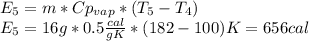
Finally, the total energy required is: