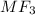Answer:
The correct answer is option 3.
Step-by-step explanation:
Molecular formula of the salt =
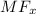
The salt when dissolved in water will get dissociated into ions.
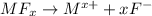
van't Hoff factor = i = 1+x
Boiling point of the solution =
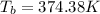
Boiling point of pure solvent that water =
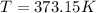
Elevation in boiling point =
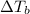
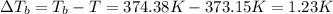
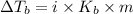
 =molal elevation constant
=molal elevation constant
m = molality of the solution
Molal elevation constant of water =0.52 K kg/mol
molality =
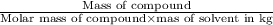

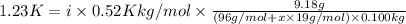
i = 2.47+0.48x
2.47+0.48x = 1+x
x =2.8 ≈ 3
Molecular formula of the salt =