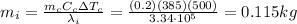Answer:
0.115 kg
Step-by-step explanation:
Since no energy is transferred to the surroundings, all the heat from the copper is used to melt the block of ice. So we can write:
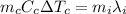
where:
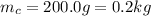 is the mass of the copper
is the mass of the copper
Cc = 385 J/kg•°C is the specific heat of copper
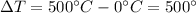 is the change in temperature of the copper (the copper stops to give heat to the ice when they are in thermal equilibrium, so when they have reached the same temperature)
is the change in temperature of the copper (the copper stops to give heat to the ice when they are in thermal equilibrium, so when they have reached the same temperature)
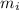 is the mass of ice
is the mass of ice
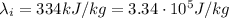 is the latent heat of fusion of ice
is the latent heat of fusion of ice
Solving the equation for the mass of the ice, we find