Answer: The correct answer is Option C.
Step-by-step explanation:
Combustion reaction is defined as the chemical reaction in which a hydrocarbon reacts with oxygen gas to produce carbon dioxide gas and water molecule. The chemical equation for this reaction follows:
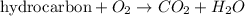
Hydrocarbon are defined as the substances in which hydrogen and carbon atoms are covalently bonded. Elements other than carbon and hydrogen can also be present.
For the given options:
- Option A:

Methane can undergo combustion reaction and produce carbon dioxide and water because it is a hydrocarbon. The chemical equation follows:
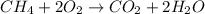
- Option B:
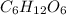
Glucose can undergo combustion reaction and produce carbon dioxide and water because it is a hydrocarbon. The chemical equation follows:
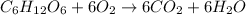
- Option C:

Hydrogen can undergo combustion reaction and will produce only water as a product. The chemical equation follows:
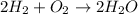
Hence, the correct answer is Option C.