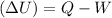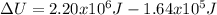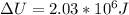Answer:
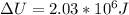
Step-by-step explanation:
due to Isobaric system, the pressure is kept constant -
Work = Pressue( V_i - V_f)
WHERE
V_i IS INITIAL VOLUME
V_f is final volume
Work = 2.0*10^5 Pa (0.824 - 1.00x10^{-3})
Work = 1.64x10^5 J
B)
increase or decrease in internal energy can be calculated by using following relation:
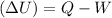
by keeping temperature and pressure constant, Q can be calculated from the phase change.
Q = (mass)(Heat of Vaporiztion)
Q = (1 kg)(2.20 *10^6) = 2.20 * 10^6 J