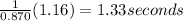Answer:
Time required is 1.33 seconds
Step-by-step explanation:
For first order reaction, the rate law expression is:
![kt = ln ([A_(0)])/([A_(t)])](https://img.qammunity.org/2020/formulas/chemistry/college/quw4322pwidqqs5hz69zy8cbq4tedgfex1.png)
Where
A0 = initial concentration = 0.830 M
At = concentration after time t = 0.260 M
t = time in seconds = ?
k = rate constant = 0.870 s⁻¹
time =
/(0.260)])](https://img.qammunity.org/2020/formulas/chemistry/college/m3gorr01m4bq79716m9ma8sd6ug2h0wz0p.png)
time =