Answer:
0.365 atm is the total pressure in the container at equilibrium.
Step-by-step explanation:
Given , equilibrium constant of the reaction in terms of partial pressure:
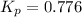
Initial Partial pressure of the hydrogen sulfide gas =
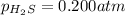
Let the partial pressure of hydrogen gas and sulfur gas at equilibrium be p.
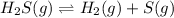
Initially 0.200 atm
At eq'm 0.200-p p p
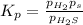
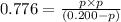
Solving the equation we will get two values of 'p' from which we will ignore the negative value.
p = 0.165 atm
Partial pressure of the hydrogen sulfide gas at equilibrium=
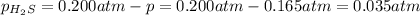
Total pressure in the container at equilibrium :
0.200-p + p + p =0.200 atm +p = 0.365 atm