Answer:
38.3958 °C
Step-by-step explanation:
As,
1 gram of carbohydrates on burning gives 4 kilocalories of energy
1 gram of protein on burning gives 4 kilocalories of energy
1 gram of fat on burning gives 9 kilocalories of energy
Thus,
27 g of fat on burning gives 9*27 = 243 kilocalories of energy
20 g of protein on burning gives 4*20 = 80 kilocalories of energy
48 gram of carbohydrates on burning gives 4*48 = 192 kilocalories of energy
Total energy = 515 kilocalories
Using,
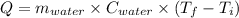
Given: Volume of water = 23 L = 23×10⁻³ m³

Density of water= 1000 kg/m³
So, mass of the water:
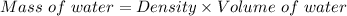
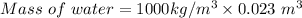
Mass of water = 23 kg
Initial temperature = 16°C
Specific heat of water = 0.9998 kcal/kg°C
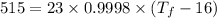
Solving for final temperature as:
Final temperature = 38.3958 °C