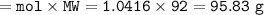Limiting reactant : O₂
Mass of N₂O₄ produced = 95.83 g
Further explanation
Given
50g nitrous oxide
50g oxygen
Reaction
2N20 + 302 - 2N204
Required
Limiting reactant
mass of N204 produced
Solution
mol N₂O :
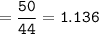
mol O₂ :
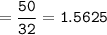
2N₂O+3O₂⇒ 2N₂O₄
ICE method
1.136 1.5625
1.0416 1.5625 1.0416
0.0944 0 1.0416
Limiting reactant : Oxygen-O₂
Mass N₂O₄(MW=92 g/mol) :