Answer:
N2O4 is a limiting reactant
Step-by-step explanation:
The first thing is to write the balanced chemical reaction
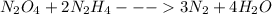
Second find the number of moles of each reactant
n(N204) =
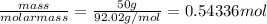
n(N2H4)=
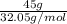 =1.4041mol
=1.4041mol
According to the stoichiometrich ratio, one mol of N2O4 two moles of N2H4 to completely react. So double. It follows therefore that that 0.54336 mol of N2O4 will require 1.0867 mol of N2H4 to completely react. BUt as we have seen here, there is more N2H4 than that, it is in excess.
Therefore
N2O4 is a limiting reactant