Answer:
7.7439×10⁻³¹ m
Step-by-step explanation:
The expression for Heisenberg uncertainty principle is:
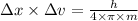
Where m is the mass of the microscopic particle
h is the Planks constant
Δx is the uncertainty in the position
Δv is the uncertainty in the velocity
Given:
mass = 0.68 g = 0.68×10⁻³ kg
Δv = 0.1 m/s
Δx= ?
Applying the above formula as:
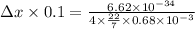
Δx = 7.7439×10⁻³¹ m