To see if mass has been conserved during a chemical reaction you must look that there is the same amount of each element on both sides.
Let's look at option A
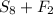 →
→
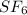
We start off with 8 Sulfurs and 2 Florine. This means that we must end up with 8 Sulfurs and 2 Florine As you can see this is not the case. We only have 1 Sulfur and 6 Florine. This means this chemical reaction is not balanced and does not show conservation of mass.
Let's look at option B
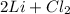 →
→
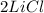
We start off with 2 Li and 2 Cl. This means that we must, again end up with 2 Li and 2 Cl. As we can see this is the case! This equation is balanced and shows conservation of mass. This is you answer!!!
I only did the work up until the answer (B.). Let me know if you need me to explain why C. and D aren't the answer.
~Just a girl in love with Shawn Mendes