Answer:
434.3727 °C
Step-by-step explanation:
Given :
The low temperature reservoir, TL = 19.5 °C
The conversion of T( °C) to T(K) is shown below:
T(K) = T( °C) + 273.15
So,
TL = (19.5 + 273.15) K = 292.65 K
Given: E₁ = 23.0 %
Let the temperature of the gas is , TH₁ = x K
The engine's efficiency of a Carnot engine is:
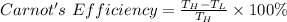
So,
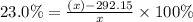
x = 379.4156 K
Now,
Given: E₂ = 64.1 %
Let the temperature of the gas is , TH₂ = y K
The engine's efficiency of a Carnot engine is:
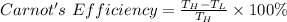
So,
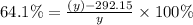
y = 813.7883 K
Also,
The conversion of T(K) to T( °C)is shown below:
T( °C) = T(K) - 273.15
x = 379.4156 K = 106.2656 °C
y = 813.7883 K = 540.6383 °C
The temperature that must be increased = 540.6383 °C - 106.2656 °C = 434.3727 °C