Answer:
Engine's Efficiency = 99.9998%
Step-by-step explanation:
Given :
The temperature of the gas is , TH = 9.5 *10⁸ K
The operation temperature of the gas is , TL = 864 °C
The conversion of T( °C) to T(K) is shown below:
T(K) = T( °C) + 273.15
So,
TL = (864 + 273.15) K = 1137.15 K
The engine's efficiency of a Carnot engine is:
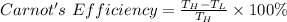
So,
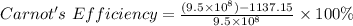
Engine's Efficiency = 99.9998%