Answer:
1-(1,1-dimethylethyl)-2-nitrobenzene from (1,1-dimethylethyl)benzene can be made in three steps as mentioned below.
Step-by-step explanation:
(1,1-dimethylethyl)benzene is sulphonated first. Here
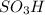 groups add onto para position to 1,1-dimethylethyl group due to electron donating effect and bulky size of 1,1-dimethylethyl group.
groups add onto para position to 1,1-dimethylethyl group due to electron donating effect and bulky size of 1,1-dimethylethyl group.
In the second step, nitration of the resulting compound is done. Here nitro group adds onto ortho position to 1,1-dimethylethyl group due to combined presence of ortho orienting 1,1-dimethylethyl group and para orienting
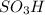 group.
group.
Then the resulting compound was heated in presence of strong acid. As sulphonation is a reversible process therefore
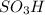 group leaves benzene ring in this condition.
group leaves benzene ring in this condition.
Schematic represention has been shown below.