Answer:
400 mL of 1.500 M HI(aq.) must be added for complete precipitation of lead
Step-by-step explanation:
Balanced reaction for formation of lead iodide-
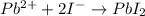
Hence, according to balanced equation, for precipitation of 1 mol of
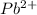 , 2 moles of
, 2 moles of
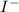 are required.
are required.
Therefore, to precipitate 0.300 moles of
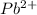 , 0.600 moles of
, 0.600 moles of
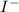 are required.
are required.
1.500 m HI(aq.) solution means 1 L of HI (or
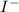 ) solution contains 1.500 moles of HI (or
) solution contains 1.500 moles of HI (or
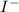 ).
).
So, volume of HI solution containing 0.600 moles
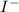 =
=
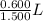 = 0.400 L = 400 mL
= 0.400 L = 400 mL