Answer:
0.003088 moles of hydrogen gas were formed .
Step-by-step explanation:
Pressure at which hydrogen gas is collected at 20°C = 768.0 Torr
Vapor pressure of water at 20°C = 17.5 Torr
Total pressure = Vapor pressure of water + Partial pressure of hydrogen gas
Partial pressure of hydrogen gas:
Total pressure - Vapor pressure of water
= 768.0 Torr - 17.5 Torr = 750.5 Torr = 0.987 atm
(1 Torr = 0.001315 atm)
Pressure of hydrogen gas =P = 0.986 atm
Temperature at which gas was collected ,T= 20°C = 293.15 K
Volume of the gas ,V= 75.3 mL = 0.0753 L
Moles of hydrogen gas = n
PV=nRT (An ideal gas equation)
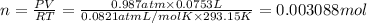
0.003088 moles of hydrogen gas were formed .