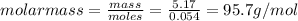Answer:
The molar mass is 95.7 g/mol
Step-by-step explanation:
As the vapor formed is showing ideal gas behavior, it will obey ideal gas law.
PV = nRT
Where
P = Pressure = 1 atm
V = volume = 1980 mL = 1.98 L
n = moles of gas
R = gas constant = 0.0821 L atm /mol K
T = Temperature = 170 °C = 170+273 = 443 K
Putting values
1 X 1.98 = n X 0.0821 X 443
n = moles = 0.054 mol
the relation between moles and molar mass and mass is:
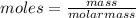
Therefore