Answer :
(a) The compound benzene follow the Trouton's rule.
(b) The compound toluene follow the Trouton's rule.
(c) The compound nonane follow the Trouton's rule.
(d) The compound chloroform follow the Trouton's rule.
(e) The compound ethanol does not follow the Trouton's rule.
(f) The compound water does not follow the Trouton's rule.
Explanation :
Trouton's rule : According to this rule, the molar entropy of vaporization should be constant that means,
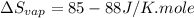
Formula used :
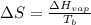
Now we have to determine the molar entropy of vaporization for the following compounds.
(a) For Benzene:
Boiling point = 353.1 K
Enthalpy of vaporization = 30.72 kJ/mole = 30720 J/mole
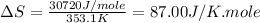
Thus, this compound is follow the Trouton's rule.
(b) For Toluene:
Boiling point = 383.6 K
Enthalpy of vaporization = 33.18 kJ/mole = 33180 J/mole
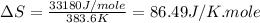
Thus, this compound is follow the Trouton's rule.
(c) For Nonane:
Boiling point = 423.8 K
Enthalpy of vaporization = 37.18 kJ/mole = 37180 J/mole
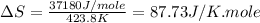
Thus, this compound is follow the Trouton's rule.
(d) For Chloroform :
Boiling point = 334.2 K
Enthalpy of vaporization = 29.24 kJ/mole = 29240 J/mole
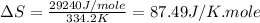
Thus, this compound is follow the Trouton's rule.
(e) For Ethanol:
Boiling point = 351.3 K
Enthalpy of vaporization = 38.56 kJ/mole = 38560 J/mole
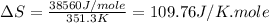
Thus, this compound is not follow the Trouton's rule.
(f) For Water:
Boiling point = 373 K
Enthalpy of vaporization = 40.65 kJ/mole = 40650 J/mole
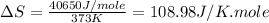
Thus, this compound is not follow the Trouton's rule.