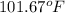Answer : The temperature of the chloroform will be,
Explanation :
First we have to calculate the mass of chloroform.
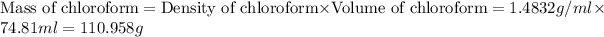
conversion used :
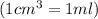
Now we have to calculate the temperature of the chloroform.
Formula used :
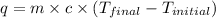
where,
q = amount of heat or energy = 1.46 kJ = 1460 J (1 kJ = 1000 J)
 = specific heat capacity =
= specific heat capacity =
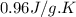
m = mass of substance = 110.958 g
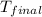 = final temperature = ?
= final temperature = ?
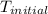 = initial temperature =
= initial temperature =
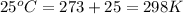
Now put all the given values in the above formula, we get:
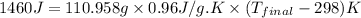
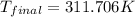
Now we have to convert the temperature from Kelvin to Fahrenheit.
The conversion used for the temperature from Kelvin to Fahrenheit is:
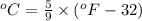
As we know that,
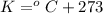 or,
or,
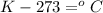
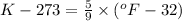
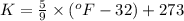 ...........(1)
...........(1)
Now put the value of temperature of Kelvin in (1), we get:
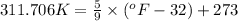
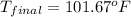
Therefore, the temperature of the chloroform will be,