Answer:
Total heat lost = 153450cal
Step-by-step explanation:
Assumptions:
- both the hypothetical life form and the scientist lose 186g of sweat per hour each
- all heat lost is due to vaporization of sweat
Heat Lost by Hypothetical Life Form
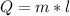
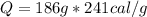
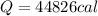
Heat Lost by Scientist
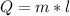
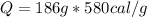
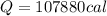
Total heat lost = 44826cal + 107880cal
Total heat lost = 153450cal