Answer:
5000 molecules of A will needed to react with 5000 molecules of B to gives 5000 molecules of AB.
Step-by-step explanation:
A+B → AB
Given that 100 molecules of A combines with 100 molecules of B to give 100 molecules of AB.
According to reaction, 1 molecule of AB is produced by 1 molecule of A.
Then 5000 molecules of AB will be given by:
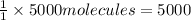 molecules of A
molecules of A
5000 molecules of A will needed to react with 5000 molecules of B to gives 5000 molecules of AB.