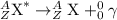Answer: a. alpha emission : atomic number decreases by 2 units and mass number decreases by 4 units.
b. beta emission : atomic number increases by 1 unit and mass number remains same.
c. gamma emission : atomic number and mass number both remain same.
Step-by-step explanation:
1. Alpha emmision: In this process, alpha particles is emitted when a heavier nuclei decays into lighter nuclei. The alpha particle released has a charge of +2 units, thus atomic number decreases by 2 units and mass number decreases by 4 units.
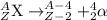
2.)Beta-emmision: In this process, a neutron gets converted into a proton and an electron releasing a beta-particle. The beta particle released carries a charge of -1 units, thus atomic number increases by 1 unit and mass number remains same.
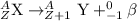
3.) Gamma ray emission: in this process, an unstable nuclei gives off excess energy by a spontaneous electromagnetic process and releases
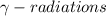 . These radiations does not carry any charge and are electrically neutral, thus atomic number and mass number both remain same.
. These radiations does not carry any charge and are electrically neutral, thus atomic number and mass number both remain same.