Answer:
7705.43 years.
Explanation:
Let us assume that initial amount of mastodon was 1.
We have been given that the radioactive element carbon-14 has a half-life of 5750 years. A scientist determined that the bones from a mastodon had lost 60.5% of their carbon-14.
We will half life formula to solve our given problem.
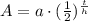 , where,
, where,
A= Final amount left after t years,
a = Initial amount,
t = time,
h = Half-life of substance.
Since the bones from a mastodon had lost 60.5% of their carbon-14, so amount of carbon-14 left would be:
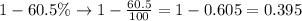
Upon substituting our given values in half'life formula, we will get:
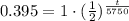
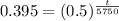
Upon taking natural log of both sides, we will get:
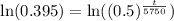
Using log property
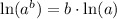 , we will get:
, we will get:
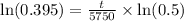
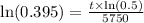
Now, we will divide both sides of our equation by
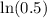 .
.
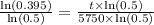
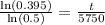
Switch sides:
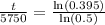
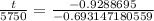
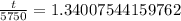
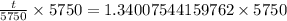
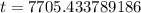
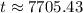
Therefore, the bones were approximately 7705.43 years old when they were discovered.