Answer:
82.8986 km
Step-by-step explanation:
Given:
Pressure = 7.00×10⁻¹³ atm
Since , 1 atm = 101325 Pa
So, Pressure = 7.00×10⁻¹³×101325 Pa = 7.09275×10⁻⁸ Pa
Radius = 2.00×10⁻¹⁰ m
Diameter = 4.00×10⁻¹⁰ m (2× Radius)
Temperature = 303 K
The expression for mean free path is:
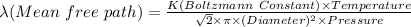
Boltzmann Constant = 1.38×10⁻²³ J/K
So,
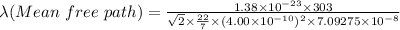
Mean free path = 82.8986×10³ m = 82.8986 km