Step-by-step explanation:
Mole percentage of carbon =
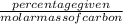
=
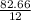
= 6.89
Mole percentage of hydrogen =
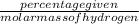
=
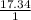
= 17.34
Now, dividing mole percentage of both the atoms by 6.89.
Then, C = 1 and H =
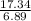 = 2.5
= 2.5
Hence, empirical formula is
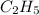 .
.
As, it is given that P = 556 mm Hg. Convert mm Hg into atm as follows.
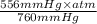
= 0.7316 atm
Volume is given as 158 mL. So, in liter volume is
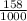 equals 0.158 L.
equals 0.158 L.
According to ideal gas equation, PV = nRT
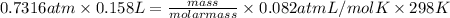
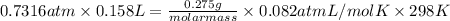
molar mass = 58.2 g
Hence, molecular weight of
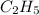 is
is
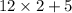 = 29.
= 29.
Therefore,
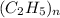 = 58
= 58
29 × n = 58
n = 2
Thus, molecular formula of the compound is
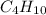 .
.