Answer:
13.6L
Step-by-step explanation:
Given parameters:
Initial Volume V₁ = 2.4L
Initial pressure P₁ = 0.85atm
Final pressure P₂ = 0.15atm
Final volume V₂ = ?
Solution
To solve the problem we have to take the condition stated in the problem very carefully. It was stated that the gas was at a constant temperature.
According to Boyle's law" The volume of a fixed mass of a gas varies inversely as the pressure changes if the temperature is constant".
We can simply apply this law to solve the problem:
The law is mathematically expressed as P₁V₁ = P₂V₂
The unknown here is V₂ the final volume. We express it as the subject of the formula:
V₂ =
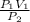
Now solving for the final volume, we have:
V₂ =
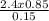
V₂ =
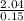 = 13.6L
= 13.6L