(a) Zero
The maximum efficiency (Carnot efficiency) of a heat engine is given by
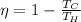
where
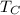 is the low-temperature reservoir
is the low-temperature reservoir
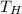 is the high-temperature reservoir
is the high-temperature reservoir
For the heat engine in the problem, we have:
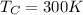
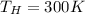
Therefore, the maximum efficiency is
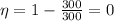
(b) Zero
The efficiency of a heat engine can also be rewritten as
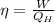
where
W is the work performed by the engine
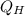 is the heat absorbed from the high-temperature reservoir
is the heat absorbed from the high-temperature reservoir
In this problem, we know
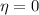
Therefore, since the term
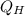 cannot be equal to infinity, the numerator of the fraction must be zero as well, which means
cannot be equal to infinity, the numerator of the fraction must be zero as well, which means
W = 0
So the engine cannot perform any work.