Answer:
2.21 moles of HCl are present in 1.2 L of a 1.84 M HCl solution
Step-by-step explanation:
Molarity (M),
Molality (m),
Normality (N),
Mass %,
Parts per million(ppm), billion(ppb), thousands(ppt) are some of the terms we use to represent the concentration of the solution that is to represent the amount of solute present in a solvent.
Molarity is moles of solute present in 1L of the solution.
The formula to find Molarity is
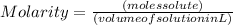 and its unit is mol/L
and its unit is mol/L
Rearranging the formula
We get
moles = Molarity × Volume
Plugging in the values
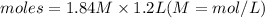
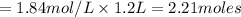 (Answer)
(Answer)