Answer:
Mg (s) is the oxidized substance and
 (g) is the reduced substance
(g) is the reduced substance
Step-by-step explanation:
If oxidation number of a substance increases then that substance is being oxidized. This is due to release of electron from that substance.
If oxidation number of a substance decreases then that substance is being reduced. This is due to consumption of electrons by that substance.
Element oxidation number
Mg(s) 0
 0 (Cl)
0 (Cl)
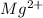 +2 (Mg)
+2 (Mg)
 -1 (Cl)
-1 (Cl)
So oxidation number of Mg increases by +2 which occurs due to release of two electrons from Mg (s). Hence Mg (s) is being oxidized.
And oxidation number of Cl decreases by -1 which occurs due to consumption of one electron by
 (g). Hence
(g). Hence
 (g) is being reduced.
(g) is being reduced.