Answer:
It will take 64.75 seconds for the concentration of NOBr to decrease to 0.025 M
Step-by-step explanation:
The integrated rate law for decomposition of NOBr is -
![(1)/([NOBr])=(1)/([NOBr]_(0))+kt](https://img.qammunity.org/2020/formulas/chemistry/college/3eppznee2plugmt3m45wwi3ndwi42ok9gg.png)
where [NOBr] is concentration of NOBr after "t" time,
![[NOBr]_(0)](https://img.qammunity.org/2020/formulas/chemistry/college/2rvydxmwwb2o1szuplcrucj9441ppmlbyf.png) is initial concentration of NOBr and k is rate constant
is initial concentration of NOBr and k is rate constant
Here [NOBr] is 0.025 M, k is 0.556
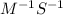 and
and
![[NOBr]_(0)](https://img.qammunity.org/2020/formulas/chemistry/college/2rvydxmwwb2o1szuplcrucj9441ppmlbyf.png) is 0.25 M
is 0.25 M
Plug in all the values in above equation-
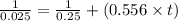
or, t = 64.75
So it will take 64.75 seconds for the concentration to decrease to 0.025 M