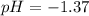Answer:
Step-by-step explanation:
We are given that 25 mL of 0.10 M
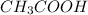 is titrated with 0.10 M NaOH(aq).
is titrated with 0.10 M NaOH(aq).
We have to find the pH of solution
Volume of
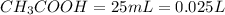
Volume of NaoH=0.01 L
Volume of solution =25 +10=35 mL=
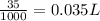
Because 1 L=1000 mL
Molarity of NaOH=Concentration OH-=0.10M
Concentration of H+= Molarity of
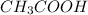 =0.10 M
=0.10 M
Number of moles of H+=Molarity multiply by volume of given acid
Number of moles of H+=
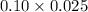 =0.0025 moles
=0.0025 moles
Number of moles of
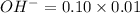 =0.001mole
=0.001mole
Number of moles of H+ remaining after adding 10 mL base = 0.0025-0.001=0.0015 moles
Concentration of H+=
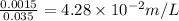
pH=-log [H+]=-log [4.28
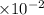 ]=-log4.28+2 log 10=-0.631+2
]=-log4.28+2 log 10=-0.631+2