Answer:
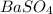 will be not soluble in water
will be not soluble in water
Step-by-step explanation:
LiOH is a strong base. Hence it gets completely dissociated in aqueous solution.
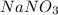 is a strong electrolyte. Hence it gets completely dissociated in aqueous solution.
is a strong electrolyte. Hence it gets completely dissociated in aqueous solution.
 is a strong electrolyte. Hence it gets completely dissociated in aqueous solution.
is a strong electrolyte. Hence it gets completely dissociated in aqueous solution.
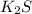 is a strong electrolyte. Hence it gets completely dissociated in aqueous solution.
is a strong electrolyte. Hence it gets completely dissociated in aqueous solution.
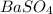 is a sparingly soluble salt. Hence it is not dissociated and hence dissolved in water. This is due to the fact that both
is a sparingly soluble salt. Hence it is not dissociated and hence dissolved in water. This is due to the fact that both
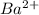 and
and
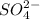 ions are similar in size. Hence crystal structure of
ions are similar in size. Hence crystal structure of
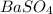 is quite stable. Hence
is quite stable. Hence
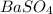 is reluctant to undergo any dissociation in aqueous solution.
is reluctant to undergo any dissociation in aqueous solution.