Step-by-step explanation:
For the given reaction
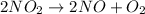
Now, expression for half-life of a second order reaction is as follows.
![t_(1/2) = (1)/([A_(0)]k)](https://img.qammunity.org/2020/formulas/chemistry/college/xt39r7pxxch53zf4q0ucggklyzepxyidhw.png) ....... (1)
....... (1)
Second half life of this reaction will be
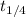 . So, expression for this will be as follows.
. So, expression for this will be as follows.
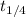 =
=
![(1)/(k) [(1)/([A]_(f)) - (1)/([A_(0)])]](https://img.qammunity.org/2020/formulas/chemistry/college/p7fqew4sv4vv18h7dvn6by3swtdfxbe71h.png) ...(2)
...(2)
where
![[A]_(f)](https://img.qammunity.org/2020/formulas/chemistry/college/nmb7zi0pxavayuu2rw1zcthtflxlivptng.png) is the final concentration that is,
is the final concentration that is,
![([A]_(0))/(4)](https://img.qammunity.org/2020/formulas/chemistry/college/cds9bexfqtu9aayrsyrdwwe4dkyrb54whs.png) here and
here and
![[A]_(i)](https://img.qammunity.org/2020/formulas/chemistry/college/5d4wcqufojo1svhg6bgxfn3tdarnx7xtqr.png) is the initial concentration.
is the initial concentration.
Hence, putting these values into equation (2) formula as follows.
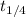 =
=
![(1)/(k) [(4)/([A]_(0)) - (1)/([A_(0)])]](https://img.qammunity.org/2020/formulas/chemistry/college/3haeg0kf9x5lxzwy1y3rhafkemk9qkumfk.png)
=
![(3)/([A_(0)]k)](https://img.qammunity.org/2020/formulas/chemistry/college/kheka6mgrjie6gwzk704upmi7eylbm454w.png) ...... (3)
...... (3)
Now, dividing equation (3) by equation (1) as follows.
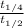 =
=
![(3)/([A_(0)]k) * [A_(0)]k](https://img.qammunity.org/2020/formulas/chemistry/college/dl49yf6n7zsituv6psio1srv7fjihqvgpx.png)
= 3
or,
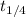 = 3
= 3
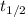
Thus, we can conclude that one would expect the second half-life of this reaction to be three times the first half-life of this reaction.