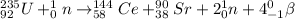Step-by-step explanation:
A nuclear fission reaction is defined as the reaction in which a heavy nucleus splits into small nuclei along with release of energy.
The given reaction is
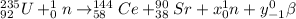
Now, we balance the mass on both reactant and product side as follows.
235 + 1 =
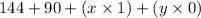
236 = 234 + x
x = 236 -234
= 2
So, now we balance the charge on both reactant and product side as follows.
92 + 0 =
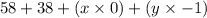
92 = 96 - y
y = 4
Thus, we can conclude that there are 2 neutrons and 4 beta-particles are produced in the given reaction.
Therefore, reaction equation will be as follows.