Answer : The equilibrium constant
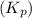 for the reaction is, 25.29
for the reaction is, 25.29
Solution : Given,
The given equilibrium reaction is,
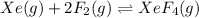
Initially 2.24 4.27 0
At equilibrium (2.24-x) (4.27-2x) x
The expression of
 will be,
will be,
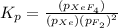
As we are given that,
The partial pressure of Xe at equilibrium = 0.34 atm
That means,
2.24 - x = 0.34
x = 1.9 atm
The partial pressure of
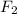 at equilibrium = 4.27 - 2x = 4.27 - 2(1.9) = 0.47 atm
at equilibrium = 4.27 - 2x = 4.27 - 2(1.9) = 0.47 atm
The partial pressure of
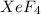 at equilibrium = x = 1.9 atm
at equilibrium = x = 1.9 atm
Now put all the given values in this above expression of
 , we get:
, we get:
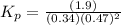
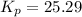
Therefore, the equilibrium constant
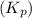 for the reaction is, 25.29
for the reaction is, 25.29