Answer: 0.011 M
Step-by-step explanation:
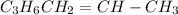
![Rate=k[C_3H_6]^1](https://img.qammunity.org/2020/formulas/chemistry/high-school/ujx658i3l82k4wei6b6tfu0k0f8iyuks7y.png)
Expression for rate law for first order kinetics is given by:
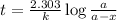
where,
k = rate constant =
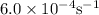
t = time taken = 525 sec
a = initial amount of the reactant = 0.0226 mol/L
a - x = amount left = ?
Now put all the given values in above equation, we get
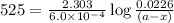
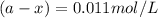
Thus the concentration after 525 s is 0.011 mol/L.